Abstract
Introduction Oncogenic activation of Wnt/β-catenin signaling is common throughout all types of cancer. Analysis of β-catenin expression in a reverse phase protein array data set encompassing 957 cancer cell lines, revealed that β-catenin protein levels are substantially lower in lymphoid tumors (110 B-, 18 T-cell leukemia/ lymphoma cell lines), while these lymphoid cells express β-catenin mRNA at similar levels to epithelial cancer cells. This suggests that mechanisms restraining β-catenin levels, such as β-catenin-degradation via Glycogen Synthase Kinase 3 Beta (GSK3β)-mediated phosphorylation, may be more active in lymphoid cells and interference with these pathways may reveal a unique dependency in lymphoid tumors.
Results To leverage GSK3β inhibition as a previously unrecognized therapeutic vulnerability in lymphoid malignancies, we examined efficacy of six different GSK3β inhibitors in a panel of cancer cell lines and patient derived xenografts (PDX). Four of the six GSK3β inhibitors (LY2090314, 6-Bromoindirubin-3'-oxime, CHIR98014 and CHIR99021) induced cell death at nanomolar concentrations selectively in lymphoid but not myeloid or epithelial cells. A combined analysis of responses to LY2090314 based on 343 epithelial cancer cell lines and 35 lymphoid cell lines revealed that IC50 values for LY2090314 were substantially higher in epithelial and myeloid cancer cells compared to B-ALL (236-fold, n=17), B-cell lymphoma (52-fold, n=7) and T-ALL (104-fold, n=11). Computational analyses of correlations between gene expression features and responses to GSK3β- inhibition across this panel of cell lines identified lymphoid specific expression of Ikaros zinc finger 1 (IKZF1) the top-ranking association.
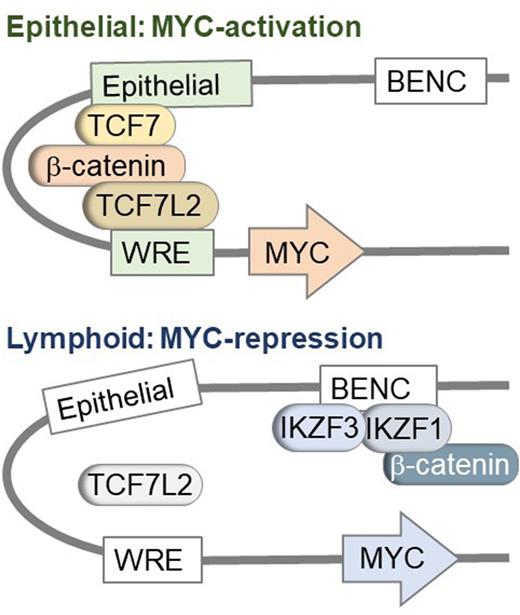
Strikingly, the four GSK3β inhibitors with lymphoid-specific toxicity (LY2090314, 6- Bromoindirubin-3'-oxime, CHIR98014 and CHIR99021) induced massive accumulation of β-catenin in parallel with acute suppression of MYC. Importantly, deletion of β-catenin conferred complete resistance of lymphoid cells to GSK3β inhibitors and relieved suppression of MYC. Similarly, inducible expression of stabilized form of β-catenin in human B- and T-cell malignancies, suppressed MYC-expression, cell proliferation and induced cell death, which indicates that accumulation of β-catenin represents the mechanistic basis of cell death upon GSK3β inhibition. In other lineages, β-catenin forms complexes with TCF7-family transcription factors to promote transcriptional activation of MYC and cell proliferation. However, our global interactome studies in B- and T-lymphocytes revealed that β-catenin complexes with lymphoid-specific Ikaros zinc finger (IKZF) proteins for transcriptional repression of MYC. Genetic deletion experiments showed that one IKZF factor was required and sufficient to enable β-catenin-driven transcriptional repression of MYC. Therefore, lesions of one single IKZF factor that are commonly found in B- and T-lymphoid malignancies would not impact sensitivity to GSK3β-inhibition.
Since LY2090314 has a demonstrated safety profile in clinical trials for patients with metastatic cancer, we tested LY2090314 as a single agent for targeted β-catenin hyperactivation in vivo in immunodeficient mice bearing patient-derived xenografts (PDX) from refractory B-ALL cells. Compared to control mice, LY2090314 substantially reduced leukemia burden and significantly extended overall survival when administered to the mice with leukemia (P=6.5E-05, n=9). This suggests that GSK3β inhibition, when used in combination with existing regimen for refractory B-ALL and other lymphoid malignancies, could substantially deepen treatment responses and improve clinical outcomes.
Conclusion GSK3β-inhibitors have previously demonstrated favorable safety profiles in phase 1 and 2 clinical trials for solid tumors, although they failed to achieve responses in micromolar serum concentrations. Importantly, our preclinical studies in B- and T-lymphoid malignancies demonstrated profound responses in low nanomolar doses. These results based on 18 clinical trials suggest that prolonged treatment at high (micromolar) concentrations is safe, however, lacks efficacy in solid tumors. Here we propose to repurpose GSK3β-inhibitors for the treatment of B- and T-lymphoid malignancies based on targeted engagement of repressive β-catenin:IKZF1 complexes.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal